Bone Growth in Inflammatory Condition
Peri-implantitis Clinical Trial
A prospective double blind randomized controlled trial that examined Pulsed Electromagnetic Field in treatment of peri-implantitis on patients that were treated with dental implants and crowns a few years ago
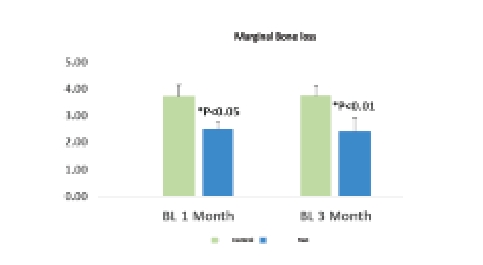

One month after treating with MED, we saw a reduction of marginal bone loss which remains constant after 3 months follow-up
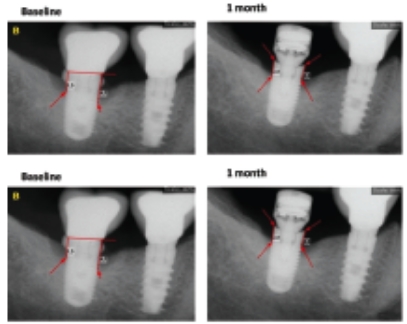
Accelerated Osseointegration
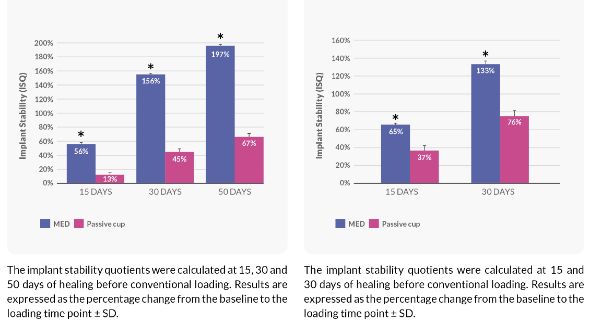
Effect of the Pulsed Electromagnetic Field (PEMF) on Dental Implants Stability: A Randomized Controlled Clinical Trial
Randomized controlled clinical trial on 40 implants placed in 20 patients
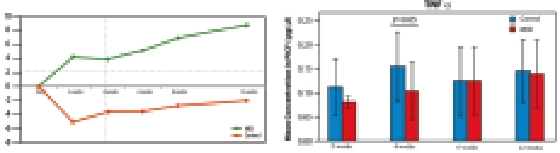
- Significant 13% increase in implant stability at test group Vs. 2% decrease in control over all time points
- 50% difference in TNFα raise in 4 weeks after implantation
- 25% difference in radiograph evaluation of marginal bone loss

Implant stability change from baseline in ISQ (Implant Stability Quotient)
Solution for high-risk patients
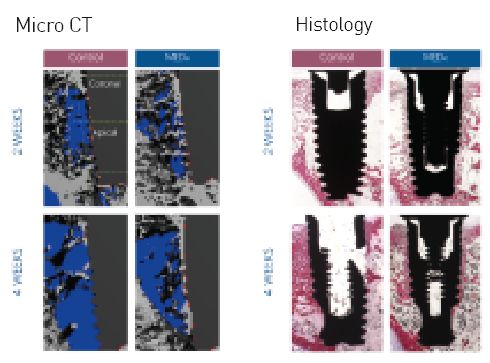
A new device for improving dental implant anchorage: a histological and micro- computed tomography study in rabbit
Controlled preclinical study on New Zealand rabbit tibia with micro-CT and histology
- 48% increase in Bone to Implant Contact (%OI)
- 62% increase in trabecular bone volume density (BV/TV)
- 73% increase in connectivity density (Conn. D)
- 44% increase in the number of trabeculae (Tb.N)
- 32% decrease in trabecular spacing (Tb.Sp)
Controlling Bacterial Colonization Around Dental Implants
Controlled in-vitro study to assess the influence of (PEMF) on Periimplantitis and connection with bacterial biofilm
Antimicrobial effects of a pulsed electromagnetic field: an in vitro polymicrobial periodontal subgingival biofilm model
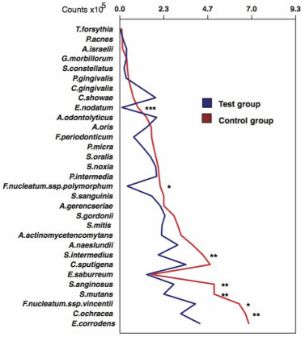
- The mean total bacterial counts were lower in the Test group vs the control group (p < 0.05)
- Antimicrobial effects on the bacterial species and can be used to control bacterial colonization around dental implants

Changes in bacterial biofilm around implant
Faster Recovery
Retrospective controlled clinical trial on 24 implants placed in 12 patients
Miniaturized Electromagnetic Device Abutment Improves Stability of Dental Implants
- Significant difference in stability rates between groups
- Similar effect at all bone types
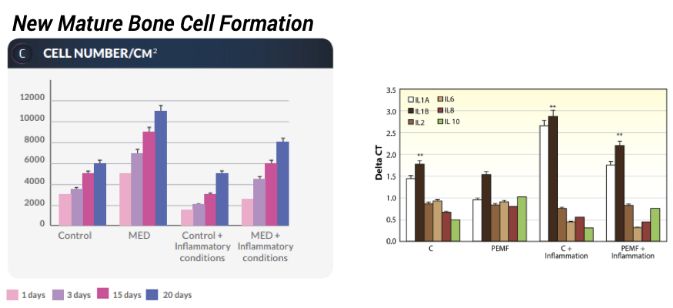
Controlled in-vitro study to assess osteoblasts formation in normal & inflammatory conditions
Pulsed electromagnetic fields increase osteogenesis commitment of MSCs via the mTOR pathway in TNF-α mediated inflammatory conditions: an in-vitro study
- Increase in MSC differentiation to osteoblasts
- Increase in MSC differentiation to osteoblasts in
inflammatory conditions - Enhanced mTOR signaling by increasing AKT, MAPP kinase, and RRAGA values
- Increase in Runx, *osteopontin, osteonectin, osteocalcin collagen type I, wnt, foxO, ALP, BMP2 and BMP7 levels
- Lower levels of *IL1B and higher levels of IL10

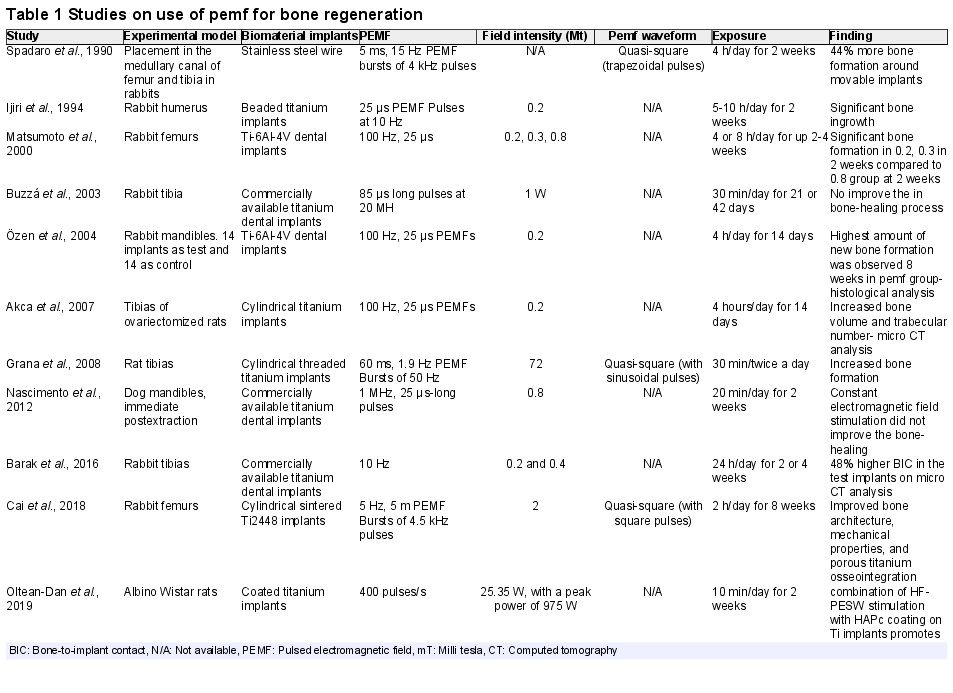
Recent literature of Pulsed Electromagnetic Fields (PEMFs) and PEMFs clinical application.
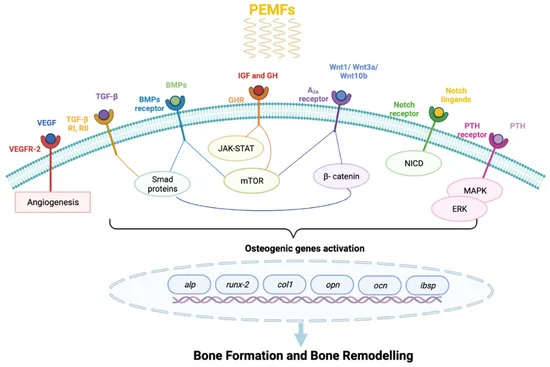
Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications
PEMFs have been widely used to enhance bone repair, accelerating healing process of recent fracture by promoting the callus formation, which can be achieved through four distinct phases: inflammatory, angio-mesenchymal, bone formation, and remodeling phases.
PEMF is a noninvasive, therapeutic form of low field magnetic stimulation that has been used for healing bone non unions and various fractures.
Biophysical therapy using the pulsating electromagnetic field as adjunctive therapy for implant osseointegration – A review.
Novel biophysical approaches that promote oral tissue healing offer various advantages due to their nonconsumable nature, ease of access to oral wounds, and efficacy of promoting the endogenous healing process that would reduce frequent patient visits and reduce the cost of overall therapy.